
Use of Axiostat Haemostatic Dressing in High-Risk Percutaneous Coronary Intervention
Percutaneous Coronary Intervention (PCI), also commonly known as angioplasty, is a medical procedure used to treat coronary artery disease (CAD) and alleviate symptoms of angina (chest pain) or to address acute coronary syndromes like heart attacks. Each year around 60 million people across the world develop a heart or circulatory disease¹ and over 3 million PCI are performed annually worldwide.² Due to the intricate nature of the treatments and the requirement for anticoagulant and antiplatelet drugs to avoid clot formation during the procedure, bleeding problems in high-risk Percutaneous Coronary Intervention (PCI) procedures are a significant concern.³
Over the last decade, there has been an expansion in the utilization of radial arteries for progressively intricate coronary procedures, including complex and high-risk rotational atherectomy-based Percutaneous Coronary Intervention (PCI) that requires the use of large-bore guiding catheters.⁴ Consequently, the topics of femoral access and the post-removal management of femoral sheath punctures are of paramount importance in cardiac catheterizations and interventions, particularly for patients at a heightened risk of complications.⁵
Over the course of several years, instances of bleeding complications have led to substantial morbidity and mortality in both civilian and military settings, during such procedures which involve vascular access sites. Despite advancements in the development of various topical haemostatic materials for various clinical scenarios over the past two decades, uncontrolled haemorrhages continue to be a global concern.³
Moreover, the latest advancements in achieving haemostasis at a radial puncture site involve the utilization of mechanical compression devices, relying on different forms of what is commonly known as “patent haemostasis” (PH). Nonetheless, the imperative need to attain any form of PH remains a priority.⁴ In recent times, Vascular Closure Devices have emerged, to facilitate the closure of puncture sites in blood vessels following certain diagnostic or interventional procedures. The main disadvantages of these device are their high price and the fact that operators need training and experience to operate VCDs safely and successfully.⁶
Talking about mechanical compression devices which use biological haemostatic component, the emerging biomaterial known as “Chitosan” is a linear polysaccharide with bioactive and mucoadhesive properties, allowing it to create a robust seal on bleeding wounds.³,⁴ This environment friendly and non-toxic material has a wide range of applications, including agriculture, food supplementation, and industrial uses. In the recent times, the market for chitosan based haemostatic dressings has significantly increased. Notably, Chitosan was initially utilized in military contexts to assist in controlling bleeding during battlefield operations.⁴
Axiostat, a haemostatic dressing that incorporates chitosan, exhibits the ability to promote swift thrombocyte aggregation and the formation of thrombus when applied to a bleeding wound.⁴ Axiostat employs the proprietary Protonated Bio adhesive Polymer technology (PBT) to make its biopolymer-based haemostats with tailorable bio adhesive properties. The highly porous structure of Axiostat helps by improving the tissue-biomaterial interaction both at macro and micro scales.⁷
Another benefit of PBT is its ability to expedite haemostasis through electrostatic interactions between the positively charged biopolymer and the negatively charged blood cells. By virtue of its positive charge, the PBT biopolymer attracts red blood cells (RBC) and platelets, capturing them within its porous structure. This, in turn, triggers platelet activation and culminates in the formation of a robust blood clot. The synergistic impact of bio adhesion and the potent blood clot results in immediate haemostasis.⁷ (Fig.1).
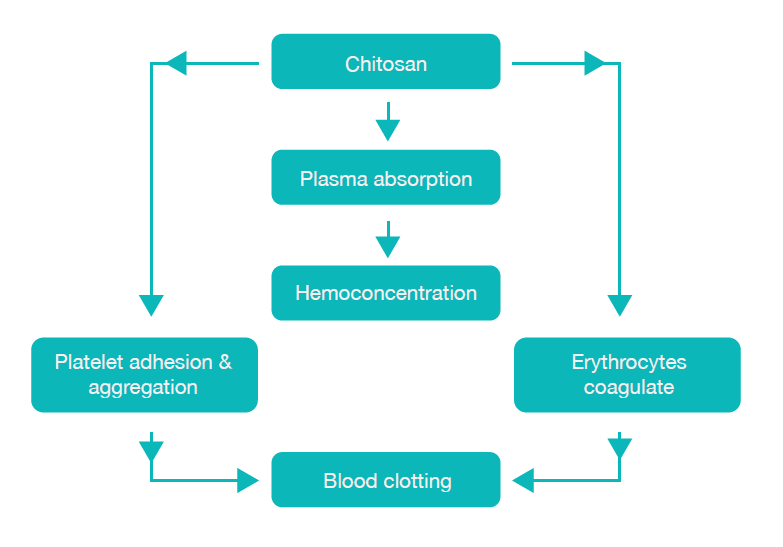
Fig: 1: Scheme of blood clotting mechanism by Chitosan
This bleeding control technique is sufficiently robust to function independently of the body’s natural clotting factors. Consequently, PBT proves effective even in patients who are using blood-thinning medications.⁷ Recently, Protonated Bio adhesive Technology (PBTᵀᴹ) technology based Axiostat dressing, was administered on radial interventional cardiology procedure for patients with high heparin dosage. Patient (50 years, Male, comorbidity – Diabetes and Hypertension) PTCA surgery was performed. The patient was administered with 12000 IU heparin dosage, and it was observed that despite the high heparin dosage the
time taken to control bleeding was significantly shorter (5 minutes) with Axiostat than with the conventional method of applying manual compression with cotton gauze, which usually takes more than 30 mins to achieve haemostasis in such cases. No rebleeding or other complications observed when peripheral area was checked after 5 mins & 15 mins.⁸(Fig.2).
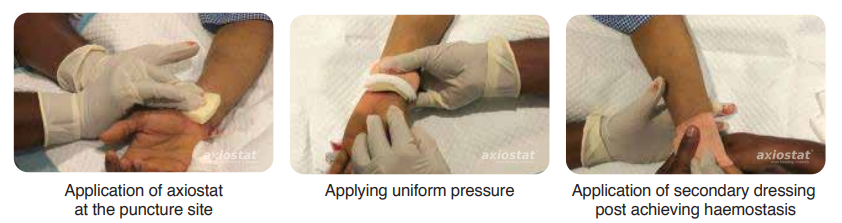
Figure 2: Axiostat use in PTCA surgery to control bleeding.
Moreover, Axiostat have been employed to reduce tourniquet durations, notably decrease pressure intervals, increase patient adherence, and shorten hospitalization periods.⁴,⁹
References
- Health Intelligence Team, B. Global Heart & Circulatory Diseases Factsheet. (2023).
- Jennings, S. et al. Trends in percutaneous coronary intervention and angiography in Ireland, 2004-2011: Implications for Ireland and Europe. IJC Heart and Vessels 4, 35–39 (2014).
- Zhong, Y. et al. Application and outlook of topical haemostatic materials: a narrative review. Ann Transl Med 9, 577–577 (2021).
- Kulyassa, P. et al. The Design and Feasibility of the: Radial Artery Puncture Haemostasis Evaluation – RAPHE Study, a Prospective, Randomized, Multicenter Clinical Trial. Front Cardiovasc Med 9, (2022).
- Byrne, R. A., Cassese, S., Linhardt, M. & Kastrati, A. Vascular access and closure in coronary angiography and percutaneous intervention. Nature Reviews Cardiology vol. 10 27–40 Preprint at https://doi.org/10.1038/nrcardio.2012.160 (2013).
- Noori, V. J. & Eldrup-Jørgensen, J. A systematic review of vascular closure devices for femoral artery puncture sites. Journal of Vascular Surgery vol. 68 887–899 Preprint at
https://doi.org/10.1016/j.jvs.2018.05.019 (2018) - Axio Biosolutions. Protonated Bio adhesive Technology. https://axiobio.com/technology/.
- AXIOSTAT CLINICAL CASE STUDIES AXIOSTAT-100% CHITOSAN HAEMOSTATIC DRESSING ON TRANSFEMORAL INTERVENTIONAL CARDIOLOGY PROCEDURE Discussion.www.axiostat.com.
- Kabeer, M., Venugopalan, P. P. & Subhash, V. C. Pre-hospital Haemorrhagic Control Effectiveness of Axiostat® Dressing Versus Conventional Method in Acute Haemorrhage Due to Trauma. Cureus (2019) doi:10.7759/cureus.5527.
 A collaborative study with Harvard Medical School
A collaborative study with Harvard Medical School