Maxiocel-FEMORAL DEEP VEIN THROMBOSIS
The main objective in addressing femoral (or iliofemoral) vein thrombosis is the prevention of blood clot formation. To achieve this, anticoagulation therapy is commonly employed to thin the blood and thwart clot development. In the realm of DVT treatment, newer medications such as edoxaban, dabigatran, rivaroxaban, and apixaban have received approval [6], [7]. In certain circumstances, the removal of a thrombus at an early stage is advisable, particularly in critical thrombosis scenario and younger patients, to mitigate the risk of long-term post-thrombotic syndromes. Presently, there are available treatment options such as endovenous procedures and surgical thrombectomy to address this [8]. Nonetheless, it is crucial to acknowledge that chronic wounds stemming from trauma or surgical procedures remains a substantial concern in the context of femoral DVT, leading to severe ramifications. Since the last decade, chitosan-based dressing has been experimentally used in dressing such wounds. Chitosan dressings offer a multifaceted approach to surgical wound care. Their inherent hemostatic, biocompatible, antimicrobial, and new granulating tissue generation properties make them valuable tools for healthcare professionals in managing various types of surgical wounds. By providing a conducive environment for healing and minimizing complications such as infections, chitosan dressings play a significant role in supporting the body’s natural wound healing processes [9], [10]. High levels of glycosaminoglycan in chitosan influence local biological activities of chitosan dressing by influencing cell-cell and cell-extracellular matrix interactions, fibroblast cell proliferation, and migration, as well as cytokine and growth factor signalling which all together favour the wound healing process in all stages [11], [12]. Recently, Bioactive microfiber gelling (BMGᵀᴹ) technology based MaxioCel dressing, was administered in femoral DVT cases. Patient (65 years, female, comorbidity – Diabetes and Hypertension) was diagnosed with massive ilio femoral DVT presented as phlegmesia cerulea dolenst and Open Venous Thrombectomy surgery was performed as an immediate treatment. Patient was suffering from extreme pain and exudate pooling from the surgical large calf wound. MaxioCel dressing was used and it showed promising wound healing results within
2 weeks (Figure 1) [13].
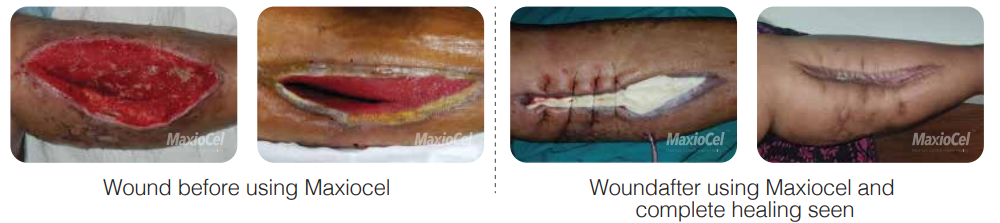
Figure 1 Usage of MaxioCel dressing in Open Venous Thrombectomy surgery Wound
References
- A. K. Jha, I. Larizgoitia, C. Audera-Lopez, N. Prasopa-Plaizier, H. Waters, and D. W. Bates, “The global burden of unsafe medical care: Analytic modelling of observational studies,” BMJ Qual. Saf., vol. 22, no. 10, pp. 809–815, 2013, doi: 10.1136/bmjqs-2012-001748.
- A. T. Cohen et al., “Venous thromboembolism (VTE) in Europe – The number of VTE events and associated morbidity and mortality,” Thromb. Haemost., vol. 98, no. 4, pp. 756–764, 2007, doi: 10.1160/TH07-03-0212.
- J. A. Heit, “The epidemiology of venous thromboembolism in the community: Implications for prevention and management,” Vein B., vol. 28, no. 3, pp. 323–330, 2007, doi: 10.1016/B978-012369515-4/50039-9.
- S. V Konstantinides, M. Mccumber, Y. Ozaki, A. Wendelboe, and J. I. Weitz, “Thrombosis: A Major Contributor to Global Disease Burden G.E. Raskob, P. Angchaisuksiri, A.N. Blanco, H. Buller, A. Gallus, B.J. Hunt, E.M. Hylek, A. Kakkar, S.V. Konstantinides, M. McCumber, Y. Ozaki, A. Wendelboe and J.I. Weitz Arterioscler Thromb Vasc ,” 2014, doi: 10.1111/jth.12698.This.
- S. H. Jeong, S. Namgoong, E. S. Dhong, and S. K. Han, “Deep vein thrombosis in donor or recipient veins encountered during lower extremity reconstruction with a free anterolateral thigh perforator flap: How do we deal with it?,” Front. Surg., vol. 9, no. September, pp. 1–13, 2022, doi:10.3389/fsurg.2022.985245.
- A. Chen, E. Stecker, and B. A. Warden, “Direct oral anticoagulant use: A practical guide to common clinical challenges,” J. Am. Heart Assoc., vol. 9, no. 13, pp. 1–18, 2020, doi: 10.1161/JAHA.120.017559.
- D. Liu, E. Peterson, J. Dooner, M. Baerlocher, and L. Zypchen, “Diagnosis and management of iliofemoral deep vein thrombosis: clinical practice guidelin,” vol. 187, no. 17, 2015, doi:10.1503/cmaj.141614/-/DC1.Recommendations.
- D. Mu¨hlberger, “Surgical thrombectomy for iliofemoral deep vein thrombosis: Patient outcomes at 8.5 years,” PLOS ONE, 2020.
- R. Singh, K. Shitiz, and A. Singh, “Chitin and chitosan : biopolymers for wound management OH,” 2017, doi: 10.1111/iwj.12797.
- M. A. Matica, F. L. Aachmann, A. Tøndervik, H. Sletta, and V. Ostafe, “Chitosan as a wound dressing starting material: Antimicrobial properties and mode of action,” Int. J. Mol. Sci., vol. 20, no. 23, pp. 1–33, 2019, doi:10.3390/ijms20235889.
- B. Farhadihosseinabadi, A. Zarebkohan, M. Eftekhary, M. Heiat, M. Moosazadeh Moghaddam, and M. Gholipourmalekabadi, “Crosstalk between chitosan and cell signaling pathways,” Cell. Mol. Life Sci., vol. 76, no. 14, pp. 2697–2718, 2019, doi: 10.1007/s00018-019-03107-3.
- H. Liu et al., “A functional chitosan-based hydrogel as a wound dressing and drug delivery system in the treatment of wound healing,” RSC Adv., vol. 8, no. 14, pp. 7533–7549, 2018, doi: 10.1039/c7ra13510f.
- “MAXIOCEL – 100% CHITOSAN WOUND DRESSING ON FEMORAL DEEP VEIN THROMBOSIS,” https://axiobio.com/uploads/pdf/maxiocel/femoral-DVT-case-study-5-maxiocel.pdf.
- H. K. Nair, “Evaluation of a novel chitosan wound healing dressing based on bioactive microfibre gelling (bmg) technologyᵀᴹ: a case series,” Wounds Asia, vol. 5, no. 3, pp. 1–23, 2022, [Online]. Available: file:///D:/Downloads/evaluation-of-a-novel-chitosan-wound-healing-dressing-based-on-bioactive-microfibre-gelling-bmg-technology-a-case-series.pdf
- “BMG Technology based MaxioCel Dressing Properties and Application,”
https://axiobio.com/maxiocel/wound-care-dressing/.
 A collaborative study with Harvard Medical School
A collaborative study with Harvard Medical School